BETHESDA, Maryland, June 6, 2016 /PRNewswire/ — Cannabics Pharmaceuticals Inc. (CNBX) filed a PCT Application with the US Patent & Trademark Office (USPTO) entitled a “System and Method for High Throughput Screening of Cancer Cells”.
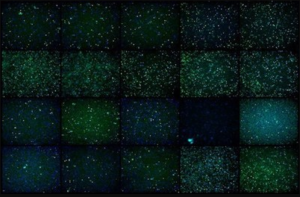
Cannabics Pharmaceuticals has developed a proprietary high throughput screening process which is designed to generate mega-data of specific cannabinoids and cannabinoid formulations with antitumor properties. In this proprietary process biopsies and live cancer cells lines are treated, In vitro, with innumerous combinations of cannabinoids and the resulting antitumor effects are screened, categorized and actually visually displayed. Dr. Eyal Ballan, Chief scientist of the company said: “This data enables doctors to provide a personalized and tailored therapy to cancer patients with the potential of reducing the tumor itself.”
Cannabics Pharmaceuticals plans to license this technology globally, wherever cancer patients have access to regulated medical cannabis. To this effect it is already in preliminary negotiations with premier oncology laboratories in Illinois, Colorado California, Oregon, as well as several other US and European jurisdictions.
About Cannabics Pharmaceuticals Inc.
Cannabics Pharmaceuticals Inc. (CNBX), a U.S based public company, is dedicated to the development of Personalized Anti-Cancer treatments. The Company’s scientific focus is on harnessing the proven therapeutic properties of natural Cannabinoids. Cannabics’ vision is to create tailored therapies for cancer patients, utilizing advanced HTS technology and personalized bioinformatics tools. The Company’s R&D is based in Israel, where it is fully licensed by the Ministry of Health and involves both scientific and academic research as well as current ongoing medical clinical studies which are registered with the US NIH.
Disclaimer:
Certain statements contained in this release constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements identified by words such as “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “targets,” “projects” and similar expressions. The statements in this release are based upon the current beliefs and expectations of our company’s management and are subject to significant risks and uncertainties. Actual results may differ from those set forth in the forward-looking statements. Numerous factors could cause or contribute to such differences, including, but not limited to, results of clinical trials and/or other studies, the challenges inherent in new product development initiatives, the effect of any competitive products, our ability to license and protect our intellectual property, our ability to raise additional capital in the future that is necessary to maintain our business, changes in government policy and/or regulation, potential litigation by or against us, any governmental review of our products or practices, as well as other risks discussed from time to time in our filings with the Securities and Exchange Commission, including, without limitation, our latest 10-Q Report filed on April 18th, 2016. We undertake no duty to update any forward-looking statement or any information contained in this press release or in other public disclosures at any time.
