TEL AVIV, Israel and BETHESDA, Maryland, Jun 28, 2021
Cannabics Pharmaceuticals Inc. (OTCQB: CNBX), a global leader in the development of cancer related cannabinoid-based medicine, released today the interim results of its second in-vivo POC study evaluating the efficacy of company’s proprietary drug candidate RCC-33 for the treatment of colorectal cancer on mice. The study objective was to evaluate the potential efficacy of the RCC-33 drug candidate as a systemic treatment for colorectal cancer when administered orally. Interim study results confirmed the potential efficacy of RCC-33 as a systemic treatment for colorectal cancer when administered orally, showing a 30% reduction in tumor volume in comparison with sham control mice, after 24 days of treatment. The results indicated statistical significance with a p-value less than 0.05.
The announcement comes following previously released in-vivo study results demonstrating a 33% reduction in tumor volume in mice treated with RCC-33 using intraperitoneal (IP) injection.
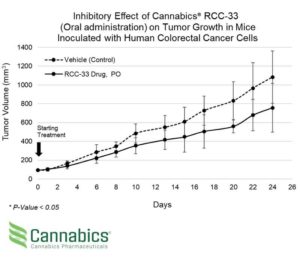
Gabriel Yariv, Cannabics Pharmaceuticals President & COO said: “Our first POC study in mice demonstrated that RCC-33 has potential anti-tumor effect on colorectal cancer. During the first POC study we used intraperitoneal (IP) administration, via injection to the abdomen, and while we were very pleased to see a 33% reduction in tumor volume, we did not know how RCC-33 would work when administered orally for systemic treatment. The leap between IP to oral administration is not a straightforward process, and it necessitated a great deal of attention. Today, after having seen the interim results, this second POC study is immensely important to us, as it reconfirms the potential of RCC-33 as a treatment candidate for colorectal cancer, as well as being efficacious via oral administration. This is another important step for us in the right direction”.
Eyal Ballan Cannabics Pharmaceuticals CTO said “These positive results move us one step closer to in-human studies. After seeing the antitumor effect reconfirmed in this POC study, our next goal is to improve the bioavailability of the drug to be administered in our upcoming clinical studies”.
Certain statements contained in this release may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other U.S. Federal securities laws. Such statements include but are not limited to statements identified by words such as “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “targets,” “projects” and similar expressions. The statements in this release are based upon the current beliefs and expectations of our Company’s management and are subject to significant risks and uncertainties. Actual results may differ from those outlined in the forward-looking statements. Numerous factors could cause or contribute to such differences, including, but not limited to, results of clinical trials and other studies, the challenges inherent in new product development initiatives, the effect of any competitive products, our ability to license and protect our intellectual property, our ability to raise additional capital in the future that is necessary to maintain our business, changes in government policy and regulation, potential litigation by or against us, any governmental review of our products or practices, as well as other risks discussed from time to time in our filings with the Securities and Exchange Commission including, without limitation, our latest 10-Q Report filed April 14th, 2021. We undertake no duty to update any forward-looking statement or any information contained in this press release or other public disclosures at any time. Finally, the investing public is reminded that the only announcements or information about Cannabics Pharmaceuticals Inc., which are condoned by the Company, must emanate from the Company itself and bear our name as its source.
